A diverse group of clinical trial participants can provide findings that are more generalizable to the entire population and also identify groups where treatment may not be effective. Both of these are important considerations when it comes to creating interventions that produce the greatest benefit for the most people.
For many years, consideration wasn’t given to the biological and physiological differences between genders, ages, races, and ethnicities when determining treatment efficacy and side effects. In some instances, crucial differences in how diverse populations respond to treatment haven’t come to light until after a product is on the market.
For example, the blood thinner Plavix was found to be less effective at preventing blood clots in African Americans with stents because this population metabolizes CYP enzymes differently. These findings didn’t surface until after the drug had been approved for use.
Diversity, which includes everything from race, ethnicity, and age to gender, socioeconomic status, and disease stage, is essential to the clinical trial industry to improve the safety and efficacy of treatments for everyone. By designing studies that are sensitive to the cultural and social norms of diverse populations, traditionally underrepresented groups can be better engaged in clinical research.
Requirements for demographic diversity
To address patient safety, the National Institutes of Health instituted a policy in 1993 that requires women and minorities to be part of any representative sample in clinical research. More recently, the Food and Drug Administration (FDA) now requires that for the results from a clinical trial to apply to the general population, the demographic mix of participants must mirror that of the entire US population.
In 2015, the FDA introduced “Drug Trial Snapshots,” a publication designed to give consumers and healthcare providers an easy to understand summary of demographic data from recently approved drugs. The intention is to increase transparency while making demographic information more available. The snapshots also include information about where clinical trials are conducted and notes if there are any differences in trial results between demographic groups.
The FDA has developed a strategy for enhancing diversity in the clinical trial process and recommends that sponsors carefully evaluate study criteria during the design phase to ensure it is as inclusive as possible. Suggestions from the FDA include a review of age, disease stage, gender, and ethnicity among participants.
The FDA also encourages Principal Investigators to consider adaptive clinical trials, which allow for pre-specified trial design changes during the trial, including altering the trial population. It’s important to note that the strategy is only a series of recommendations; there is no regulatory requirement that these are part of a clinical trial.
Understanding minority underrepresentation in clinical research
Historically, racial and ethnic minorities have been underrepresented in clinical trials, and this trend continues in modern studies. In 2011, it was reported that African Americans and Hispanics made up 12% and 16% of the US population, respectively, and yet they only accounted for 5% and 1% of clinical trial participants.
To further illustrate the importance of diverse populations, consider that the asthma medication Albuterol was found to be less effective in African American and Puerto Rican children when compared with European American and Mexican children. Or that the prostate cancer drug Zytiga was found to be more effective in African American men than Caucasian men in post-market studies conducted by the trial sponsor.
In many instances, diverse participants weren’t recruited to participate because of time and cost considerations. Recruiting a diverse sample that meets inclusionary criteria can take considerably longer than looking at the general population for participants. However, addressing this issue now is vital because as demographics continue to change, estimates show that 50% of the US population will be other than a non-Hispanic white by 2045.
While these shifting demographics may pose a challenge there are ways to address underrepresentation head on. Through the effective use of social media, recruiting qualified participants from diverse backgrounds is much easier. In our experience, the use of targeted advertising while running concurrent campaigns that can be adjusted in real time is an effective way to boost minority representation, often at no additional cost to trial sponsors.
Strategies to improve diversity
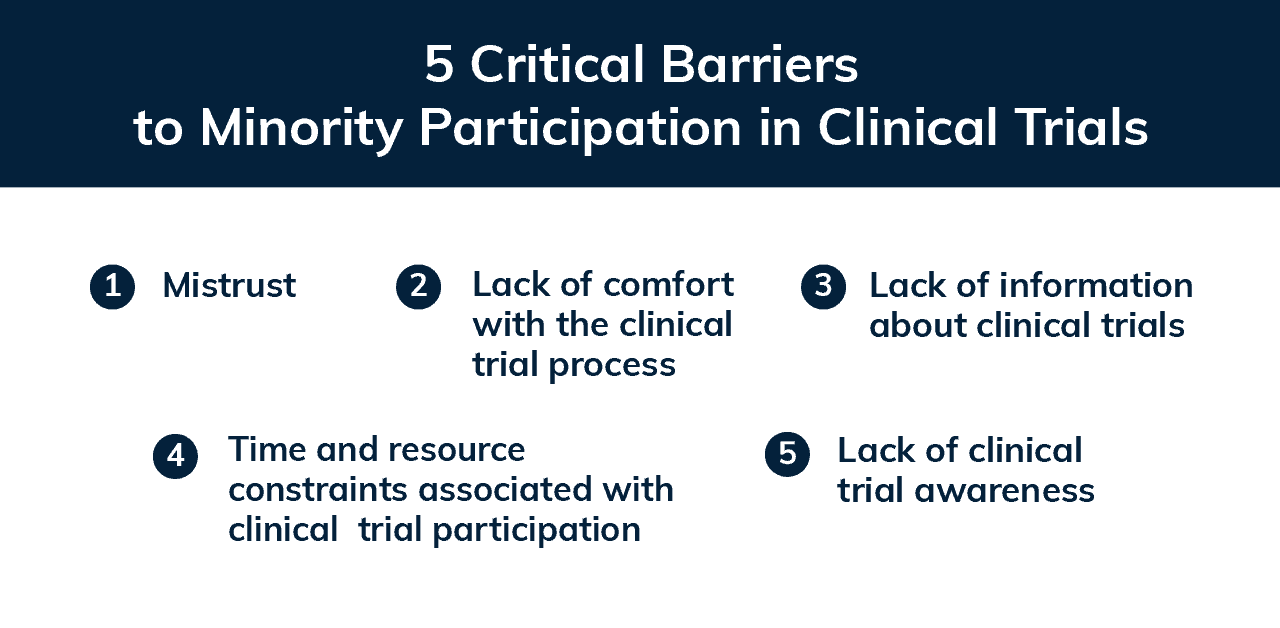
Research has identified five main barriers to minority participation in studies. These include mistrust, including a lack of information about and comfort with the research process, logistical obstacles like time and resource constraints, and limited clinical trial awareness. Additionally, cultural competency and knowledge among study staff is often an ongoing issue, but there are actionable ways to address these concerns.
Focus on building relationships
Successful recruitment for a clinical trial depends on quality relationships between study staff and participants. Study teams can make the entire process run more effectively by focusing on excellent communication, setting and meeting expectations, and creating simple processes for participants to follow. These approaches build trust and reduce the likelihood of participants feeling as though their time and contributions to the study aren’t valued.
When working with diverse groups of participants, it’s important to remember that they may have perceptions of clinical trials that are very different from that of the study team. There could be a historical legacy of mistrust between patients and healthcare providers that influences their willingness to participate. Bridging this gap is a sensitive issue and requires awareness of cultural and social influences.
The best example of this is the Tuskegee Study of untreated syphilis. Starting in 1932, the Public Health Service and the Tuskegee Institute began an exploration of the natural progression of untreated syphilis in African American men. Participants weren’t told what the study was about in enough detail to provide informed consent, nor were they offered treatment even when penicillin became the treatment of choice in 1947.
The study was intended to last six months but actually lasted 40 years. The legacy of the Tuskegee Study has resulted in significant mistrust between the African American community and the healthcare and research industries that is still relevant today.
Increasing cultural competency
One way to improve understanding of important social and cultural influences among participants is through cultural competency training. Cultural competency goes beyond a general awareness of the differences between racial and ethnic groups. It is also more than linguistic barriers or genetic variations between participants.
It includes social and environmental variations like socioeconomic status, the role of family members and caregivers in decision making, and general feelings held by individuals towards healthcare services and providers.
Sponsors and study teams can improve their cultural competency by:
- Educating themselves about the differences between racial and ethnic groups and the general population that may influence decision making.
- Developing diverse participant advisory groups that provide direct feedback to study teams.
- Training staff in cultural awareness, sensitivity, and cultural safety best practices.
- Learning about effective ways to recruit minority or representative samples.
Research published in 2019 suggests that “…patients are most concerned with obtaining the best treatment for their disease… Therefore, reassuring patients about the importance of their health and safety, reinforcing that their health will be closely monitored, and including family members in decision making should increase comfort [with participation]…” The inclusion of cultural competency will also help build trust between study teams and participants, that encourages effective relationship building.
Actionable ways to improve diversity in clinical trials
Sponsors and study teams can improve diversity and inclusion by working directly with potential participants during the design phase of a study and incorporating their feedback. This can be done by:
- Connecting with community groups (places of worship, schools, community associations, etc.) around clinical trial sites to offer presentations and information sessions for residents.
- Creating a participant advisory team that is representative of the proportion of the disease under study within a particular demographic group.
- Identifying key community leaders and asking for feedback.
- Designing an online survey to describe the study and ask for feedback about areas of the study design.
- Considering clinical trial sites located in areas with a higher concentration of racial and ethnic minority patients to recruit a more diverse study population.
- Using Trialfact’s resources on recruiting representative samples.
When identifying healthcare providers to help with clinical trial recruitment, diversity is also important because this may promote diversity among participants. It may be valuable to work with minority physicians to run clinical trials because some patients prefer being treated by physicians of their race, ethnicity, or gender. Strategies to engage these providers include:
- Approaching minority physicians in the area around a clinical trial site about their potential involvement.
- Identifying minority physician thought-leaders on social media, including Twitter and LinkedIn, and asking for their input.
- Engaging with diverse physician groups like the National Medical Association (representing African-American physicians and patients), the American Medical Women’s Association, or the National Hispanic Medical Association.
Tackling the challenge of diverse representation in clinical trials is vital for the health and safety of everyone. Through the inclusion of different races, ethnicities, genders, and age groups during a clinical trial, the results from the study are more generalizable to the population because they reflect current demographics.
Trialfacts has recruited for 100s of clinical trials across multiple disease areas using social media, and has developed expertise in best practices for recruiting representative samples. Additionally, our Due Diligence process brings predictability and consistency to the entire recruitment process and can help you overcome challenges with participant diversity. Schedule a call with us today to find out more about how we can help.