If you are ready to see how many participants we can guarantee for your study, request your free, no-obligation recruitment plan today. Click the button below and fill out the 30 second form to get started.
 If the work of research team members Loredana Esposito, Mario Nogueira, and Sarone Coetzee is successful, it could help millions of people worldwide who suffer from sleep apnea have a safe and peaceful sleep.
If the work of research team members Loredana Esposito, Mario Nogueira, and Sarone Coetzee is successful, it could help millions of people worldwide who suffer from sleep apnea have a safe and peaceful sleep.
The three researchers at Bayer Pharmaceuticals are conducting a randomized controlled trial to help sleep apnea patients manage their symptoms with a new medication.
But their Phase 1 trial presented this trio more challenges than they’d bargained for. They faced tight timelines, a new enrollment site, unexpected response levels, and a global pandemic.
The team would need to work fast, develop a creative recruitment strategy, and take a few risks to hit their enrollment goals and complete a successful Phase 1 study.
In February 2020, Loredana, Mario and Sarone started the KOALA clinical trial, a drug safety study for patients with Obstructive Sleep Apnea (OSA).
The KOALA trial was aiming to answer two questions:
“The first question,” explains Mario, “was if the drug we were testing provided a change in the patient’s breathing overnight.” The group was looking to assess if there were any adverse effects in patients when taking the new drug.
The second question was if the drug was administered to patients differently – either by spray, drops, or another method – would they see different results?
The team had defined their study’s success, but recruitment was proving to be much more challenging than they had anticipated.
As they continued to outline the logistics of the trial, it was clear that there would be more challenges than expected.
While the group was well-versed in other therapeutic areas and has decades of combined experience, none of them had conducted a sleep apnea study before. “Our experience in this therapeutic area was limited,” Loredana says.
As with many studies, there was pressure to get started. The timeline was tight and there were only a few months to find participants and conduct their research.
Any delays in this initial phase would ripple out into the later phases of the trial.
“At the beginning, the site wanted to use their own database and the recruitment of patients that they had established, but they quickly saw that it wasn’t going to be successful.”
Mario Nogueira
Bayer Pharmaceuticals
Furthermore, the team had not worked with this particular research site before and were unsure of the number of participants they could provide.
Complicating things further, the enrollment site for the early Phase 1 study had moved from Sydney to Adelaide, Australia, which was a new region for the researchers.
“At the beginning, the site wanted to use their own database and the recruitment of patients that they had established,” Mario says “But they quickly saw that it wasn’t going to be successful.”
The site had also tried some traditional advertising techniques, including display posters, around the research site’s local area. Still, nothing was turning out to be as successful as everyone had hoped.
Ultimately, the research site wasn’t entirely confident they would reach the required number of enrollments based on their database. Instead, the team needed to set up a “safety net.”
If the research site recruitment failed, they would have a back-up ready.
“It was risky. We didn’t want to take a chance. We needed a Plan B,” Loredana says. “The life of the program depended on this trial. If there were any delays with this study, the entire program would be delayed as well.”
Although the research team was aware of recruitment companies, they had worked with one previously and their overall experience was far from ideal.
“The previous vendor we worked with provided one or two patients, and they were not eligible,” explains Sarone. As a result, the team was hesitant to give recruitment outsourcing another shot.
The worry was that they’d waste time and money working with another recruiter just to see poor results again. With such tight timelines, taking a big chance like this was risky.
“Our biggest fear was that Trialfacts wouldn’t deliver,” says Loredana.
“If I’m honest, deciding to work with Trialfacts came down to two reasons: One, you came highly recommended by sites. And two, you offered me a guarantee. I didn’t have much to lose.”
Sarone Coetzee
Bayer Pharmaceuticals
Despite their reservations, Sarone was willing to give Trialfacts a try.
“If I’m honest, deciding to work with Trialfacts came down to two reasons: One, you came highly recommended by sites. And two, you offered me a guarantee. I didn’t have much to lose,” she says.
The other factor that came into play when deciding to work with Trialfacts was the recruitment forecasting and money-back guarantee.
The research team was running out of options. They had to find participants so this study, and future studies for the drug in Australia, would succeed. They decided to give Trialfacts a shot.
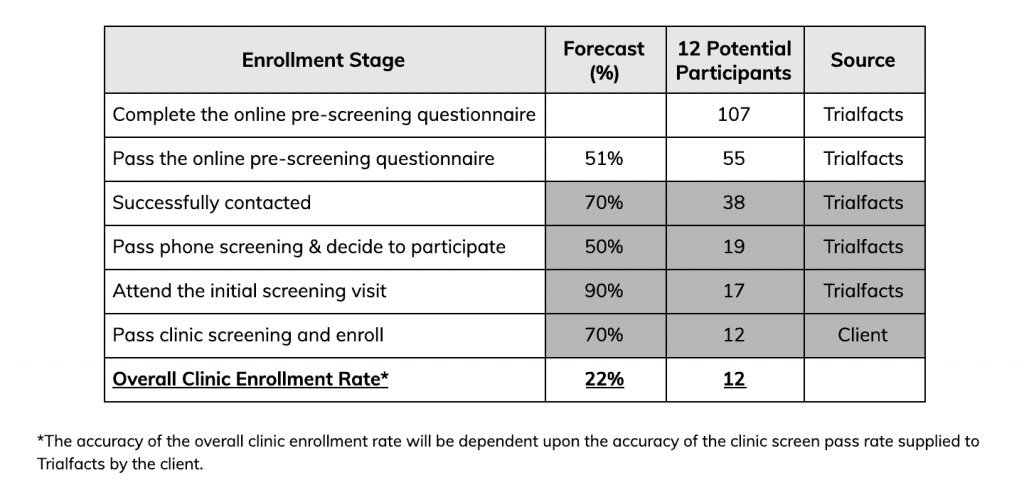
The team began recruiting with Trialfacts in February 2020. They had two months to recruit for the study and needed 12 participants enrolled to complete the first phase of the trial.
“We thought we’d be getting one, two, three patients per week, but in one month, we got 56 patients completing the online prescreen questionnaire.”
Mario Nogueira
Bayer Pharmaceuticals
As soon as they started with Trialfacts, however, the research team had a whole new challenge on their hands.
“When the campaign started, no one was expecting it to be that successful,” Mario tells us.
“We thought we’d be getting one, two, three patients per week, but in one month, we got 56 patients completing the online prescreen questionnaire.”

The results were far beyond what the team at Bayer had expected but the campaign’s success created its own set of issues.
“The limiting enrollment factor wasn’t Trialfacts or the patients Trialfacts had provided,” Mario clarifies. “It was logistics.”
Since the trial was focused on studying the participants while sleeping, the primary site staff were sleep technicians who worked in the evenings. They were not available at regular working hours to process the prospective participants which complicated things and created a backlog of potential participants.
“There had to be an entire operation of coordinating phone screening of new patients and sleep visits with the other patients,” Mario says.
Managing the large volume of patients required the research team to make some changes.
First, the site staff created a schedule to handle all of the incoming calls.
Then, they added a secondary phone screening and worked with Trialfacts to further narrow each participant’s eligibility. “One of our concerns was that our initial inclusion/exclusion protocol was too broad.”
Finally, they filtered the participant pool further by making sure potential recruits could stay overnight, were comfortable sleeping face up, and were already using a CPAP machine.
The updated requirements ensured that all potential participants were thoroughly vetted and would save the research site time with phone screening.
“Participants were keen to participate in something that would help them not use the CPAP machine because it’s very uncomfortable for them,” Mario explains.
With adjustments in place, the team was on their way to meeting their recruitment goals.
Then, COVID-19 arrived. The enrollment site locked down at the end of March and the Trialfacts campaign came to a halt.
“We had screened 10 patients and had 6 patients enrolled and treated, which was half of what we needed. I was checking the spreadsheets each day and seeing the participants come in, in real-time, it was great.”
In May, however, when the site reopened, the team found themselves in a good position.
The overwhelming response to the earlier Trialfacts prescreen questionnaires allowed them to get their additional participants without resuming the recruitment efforts. They used the participant database from the pre-COVID shutdown to finish filling the study.
“In two months, we already had 50 prospective participants. So, when the site came back in May, we only needed 6 extra participants to complete the study and used the previous questionnaires to fill those spaces,” says Mario.
By the end of July, the team had all the patients they needed to complete the first part of the study.
In the end, the team’s “Plan B” became “Plan A.”
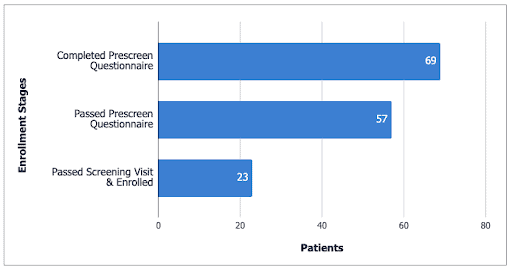
“We didn’t realize how effective a Facebook campaign could be. We had a huge number of patients that answered the online prescreen questionnaire – 57 people passed online screening – and we only needed 12. In the end, there were 23 enrolled and 12 treated,” Loredana says.
“Only 6 of the 23 enrollments came from the site’s database. The rest came from Trialfacts. It’s quite a success story,” Sarone adds.
The large pool of participants from Trialfacts offered an added benefit.
“We knew that if we needed an extra patient for Phase 2, we had them. This made us calmer and more secure that we wouldn’t have to stress about finding someone,” Mario shares.
“If it weren’t for Trialfacts, we wouldn’t have met our timelines. Full stop. We say that every day. We throw you on the table for every new study we get served. We punted and it worked.”
Loredana Esposito
Bayer Pharmaceuticals
The pressure was on, and the team came through brilliantly.
“The simplest way to say it is, if it weren’t for Trialfacts, we wouldn’t have met our timelines. Full stop. We say that every day. We throw you on the table for every new study we get served. We punted and it worked,” Loredana says.
“If we didn’t use Trialfacts, we would not have met our timeline. We say that every day.
“We have just finished a feasibility proposal for an upcoming study and your support helps us put a really good bid in for numbers when it comes to future participants for other studies as well.”
The group sees the ability to use external recruitment as a safety net for their future studies. “It’s about making sure that we meet our commitment as a country, so we stay on the map when it comes to trials,” adds Loredana.
With the Phase 1 successes under their belt, this resourceful team has moved on to the Phase 2 trial. Things are going smoothly and they are now ready to start a feasibility study in December.
“We’re now moving to Phase 2, and we will be looking to you to help us!” Loredana says, smiling.
The KOALA research team overcame one obstacle after another to get their study where it is today and are well on their way to a successful trial.
Their results will have a great impact helping patients get a good night’s sleep.

If you are ready to see how many participants we can guarantee for your study, request your free, no-obligation recruitment plan today. Click the button below and fill out the 30 second form to get started.

Paratus Clinical recognized the need for a high ROI as they began to invest in clinical trial patient recruitment. They needed to recruit as many participants as possible while using their sponsor’s funding wisely.