Please note: This trial has finished recruiting and is not accepting new participants.
Research Center: Skin Health Institute Inc.
Location: 1/80 Drummond St, Carlton VIC 3053
Principal Investigator: Associate Professor Peter Foley
Ethical aspects of this study have been reviewed and approved by Bellberry Human Research Ethics Committee
Background
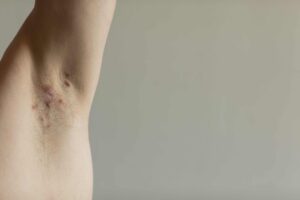 Hidradenitis suppurativa (HS) is a painful and long-term skin condition. It occurs near hair follicles where sweat glands (usually around the groin, bottom, chest, and armpits) may cause swelling, scarring, boil-like lumps, and a buildup and leak of pus in the skin. Researchers are focusing on ways to develop effective treatments for this skin condition.
Hidradenitis suppurativa (HS) is a painful and long-term skin condition. It occurs near hair follicles where sweat glands (usually around the groin, bottom, chest, and armpits) may cause swelling, scarring, boil-like lumps, and a buildup and leak of pus in the skin. Researchers are focusing on ways to develop effective treatments for this skin condition.
This study seeks to determine the safety and effectiveness of a potential new treatment for hidradenitis suppurativa (HS). Through this study, people suffering from hidradenitis suppurativa (HS) may be presented with a new possible treatment option.
Why Participate?
- You may receive a potential new treatment for your hidradenitis suppurativa.
- You may experience improvements to your HS signs and symptoms, although a direct benefit cannot be guaranteed.
- You may be helping others who also have hidradenitis suppurativa (HS).
- You will be compensated for your travel and expenses for participating in this study.
- You will be helping to advance skin health research.
Your Rights
- If you decide to participate in the study and later feel that you no longer wish to be part of it, you may withdraw at any time without any negative consequences or loss of benefits.
- Your records relating to this study and any other information received will be kept strictly confidential, except as required by the law.
- Qualified health professionals will monitor your health as it relates to the study.
Who Can Participate?
- Men and women aged 18 years old and above who are diagnosed with hidradenitis suppurativa (HS)
- Must be able to attend study visits every 2 weeks as well as comply with other study requirements for up to 73 weeks (about 16 months)
Please note:
Due to the current situation with COVID-19 pandemic, the study team would like to point out the following:
- The research site does not treat COVID-19 patients and/or suspected COVID-19 patients.
- The research site’s waiting area is spacious enough that participants will not be within close proximity to each other.
- Hand sanitisers are available at the research site.
- The research staff adheres to strict hygienic practices as well as other safety measures to prevent the spread of COVID-19.
- Any staff suspected to have COVID-19, or has come into contact with someone who has COVID-19, will automatically undergo a voluntary quarantine for 14 days.
- Interested participants will not be left waiting for a long period of time and will be quickly attended to.
- Street parking and public car parks available in the vicinity of the Institute.
- The study team encourages the use of personal vehicles, taxi or rideshare services rather than public transit.
- The research site/institution is taking all precautionary measures to ensure the safety of the study participants.