In this post we summarize current industry guidance so you can create effective compensation plans for your study. By bringing more consistency and transparency to participant compensation, managing the ethical and practical considerations becomes easier. Ultimately, a well designed compensation plan should help you boost enrollment, save time, reduce costs and improve your study completion rates.
Questions about participant compensation in clinical trials come up all the time. You’re not alone if you’ve ever asked:
- Is it ethical to offer money for participating in a clinical trial?
- Should I pay research participants for their time?
- How much should I pay a research participant?
- What kind of costs should I reimburse a research participant for?
The answers to these questions often come with their own set of practical and ethical challenges that you might not initially think about. This is further complicated because there are some guidelines in the industry, but no one size fits all approach that can be followed by sponsors. This post will help you create an action plan that fits with your study and is mindful of current practices across the industry.
Ethical implications of trial compensation
When looking strictly at compensation for participation, there are ethical concerns about the undue influence this may have over the integrity of trial results. There’s concern that participants may be coerced or lack the capacity to make an informed decision about trial participation. If there is enough of an incentive, some might withhold information or be motivated to make up a fake health history.
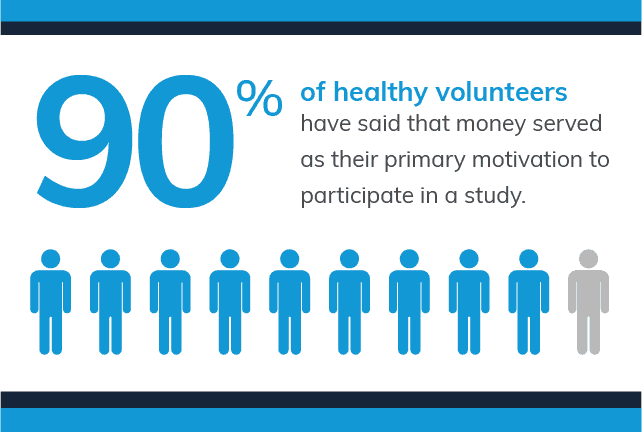 These challenges typically result in Institutional Review Boards (IRB) and other regulators to insist on compensation being minimal to prevent these types of situations. This concern isn’t without justification as research shows that offering money often increases response rates and willingness to participate. When recruiting healthy volunteers, up to 90% have said that money served as their primary motivation.
These challenges typically result in Institutional Review Boards (IRB) and other regulators to insist on compensation being minimal to prevent these types of situations. This concern isn’t without justification as research shows that offering money often increases response rates and willingness to participate. When recruiting healthy volunteers, up to 90% have said that money served as their primary motivation.
What’s important to understand is that there is a difference between coercion and undue influence. According to Christine Grady from the Department of Bioethics at the National Institutes of Health Clinical Centre, “Coercion is a threat of physical, psychological, or social harm in order to compel someone to do something, such as participate in research. Money is an offer or an opportunity and not a threat of harm.”
Undue influence is an “offer that can’t be refused” and is strong enough to compel someone to participate even when it’s against their best interests. Offering excessive compensation that leads people to make poor judgments about their participation is something to avoid. Sponsors should be aware of this during study design and when seeking IRB approval.
Practical considerations of participant compensation
Based on our experience, compensating participants for their time typically results in higher enrollment numbers, fewer drop-outs, and can reduce study timelines. We’ve seen both a real and perceived impact of compensation on enrollment and retention in the studies that we’ve recruited for.
However, there are many practical factors including the administrative resources needed to process and deliver the compensation, gather tax information including social security numbers, collect postal addresses, and follow up to ensure that the compensation was received.
Sponsors need to be aware that any monetary compensation may have tax implications for participants, and each study site might be responsible for collecting and storing tax information for each participant.
There are also situations where participants who receive government assistance like welfare or disability benefits may have their monthly benefit reduced. For example, if a participant accepts a check for $100 once a trial is over and it’s reported as income, they may find their monthly government benefit reduced by $100. The additional burden this puts on individuals who are already vulnerable raises concerns that sponsors need to consider when creating a compensation plan.
Learning when to offer compensation
Without standards across the industry, trial sponsors must make these decisions independently. Offering some guidance, the Council for International Organizations of Medical Science (CIOMS) and the World Health Organization (WHO) state that compensation and reimbursement should always be offered for participation in both intervention and observational studies. Even in situations where there are potential health benefits to the participant, payment for the inconvenience of involvement is suggested.
CIOMS states that compensation is directly related to the time and inconvenience that study participation requires, not the risk associated with participation. High-risk studies should not offer compensation that risks undue influence, as this could cause individuals to make poor judgments about their involvement.
Research participants should be reasonably reimbursed for costs directly incurred during the research, such as travel costs, and compensated reasonably for their inconvenience and time spent. Compensation can be monetary or non-monetary. The latter might include free health services unrelated to the research, medical insurance, educational materials, or other benefits. Compensation must not be so large as to induce potential participants to consent to participate in the research against their better judgment (“undue inducement”). A local research ethics committee must approve reimbursement and compensation for research participants.
These guidelines can serve as the basis for trial sponsors to develop practices around compensation. By working proactively during study design, compensation can be factored in as a enrollment expense, and its impact on participation can be evaluated. In all situations, it’s necessary to get IRB or other ethics approval of the compensation plan before the study starts.
Delivering payment to participants
The actual delivery of payment to participants may need a lot of administrative support from site staff. Assigning these tasks to a research coordinator or administrative assistant without fully understanding the time associated with the process can lead to delayed payments, and it may jeopardize the relationship between the participant and study site.
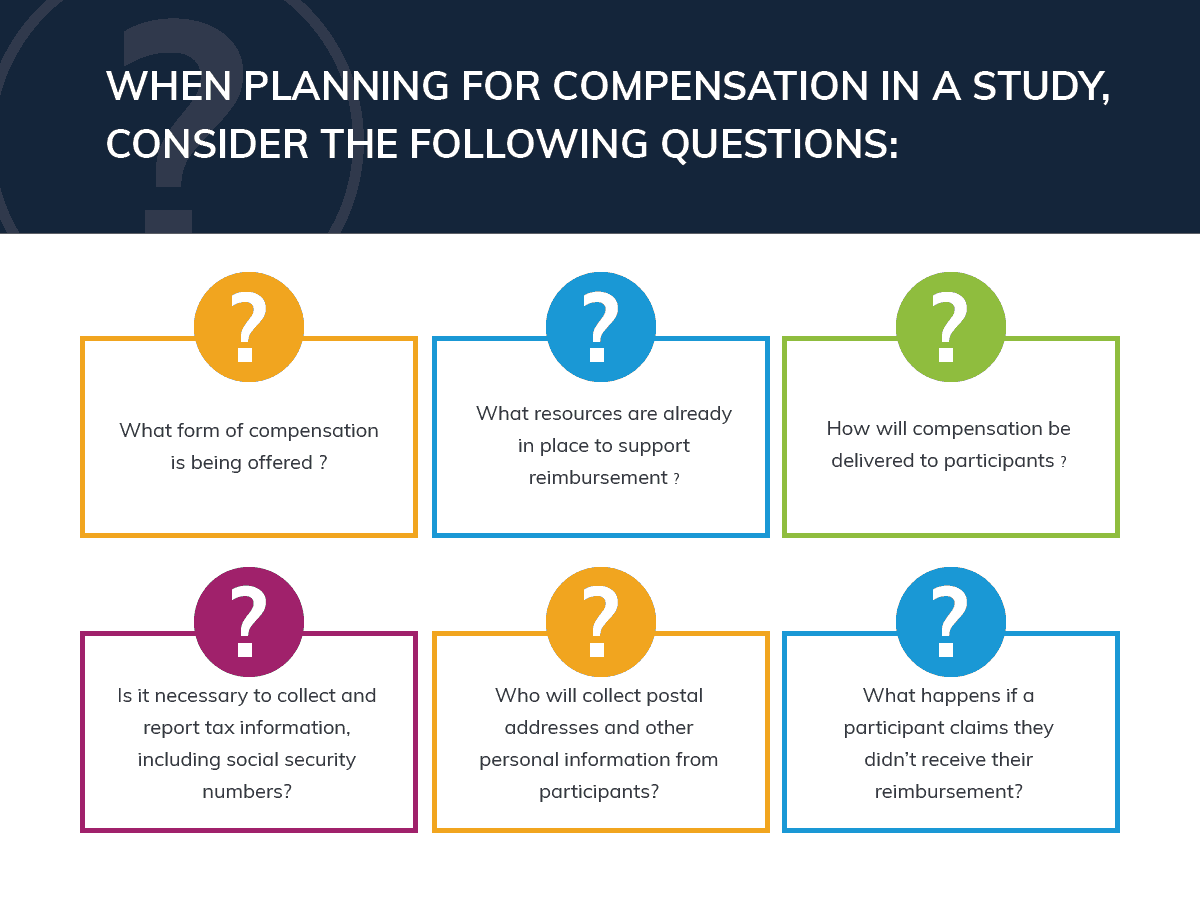
When planning for compensation in a trial, consider the following questions:
- What form of compensation is being offered (cash or check, physical, or electronic gift cards)?
- What resources are already in place to support reimbursement (existing Accounts Payable departments, specific processes, etc.)?
- How will compensation be delivered to participants (mail, email, or in-person)?
- Is it necessary to collect and report tax information, including social security numbers?
- Who will collect postal addresses and other personal information from participants?
- What happens if a participant claims they didn’t receive their reimbursement?
The size and complexity of a study will also have implications as these factors will influence the time and resources needed to deliver compensation. An online survey with 1,000 participants will take considerably longer to complete than a small intervention study with only 20 people.
Analyzing the different compensation models
 Regardless of what’s offered to participants, there is still the question of how much compensation should be worth. Several models are used across the industry that can help when making decisions. These include:
Regardless of what’s offered to participants, there is still the question of how much compensation should be worth. Several models are used across the industry that can help when making decisions. These include:
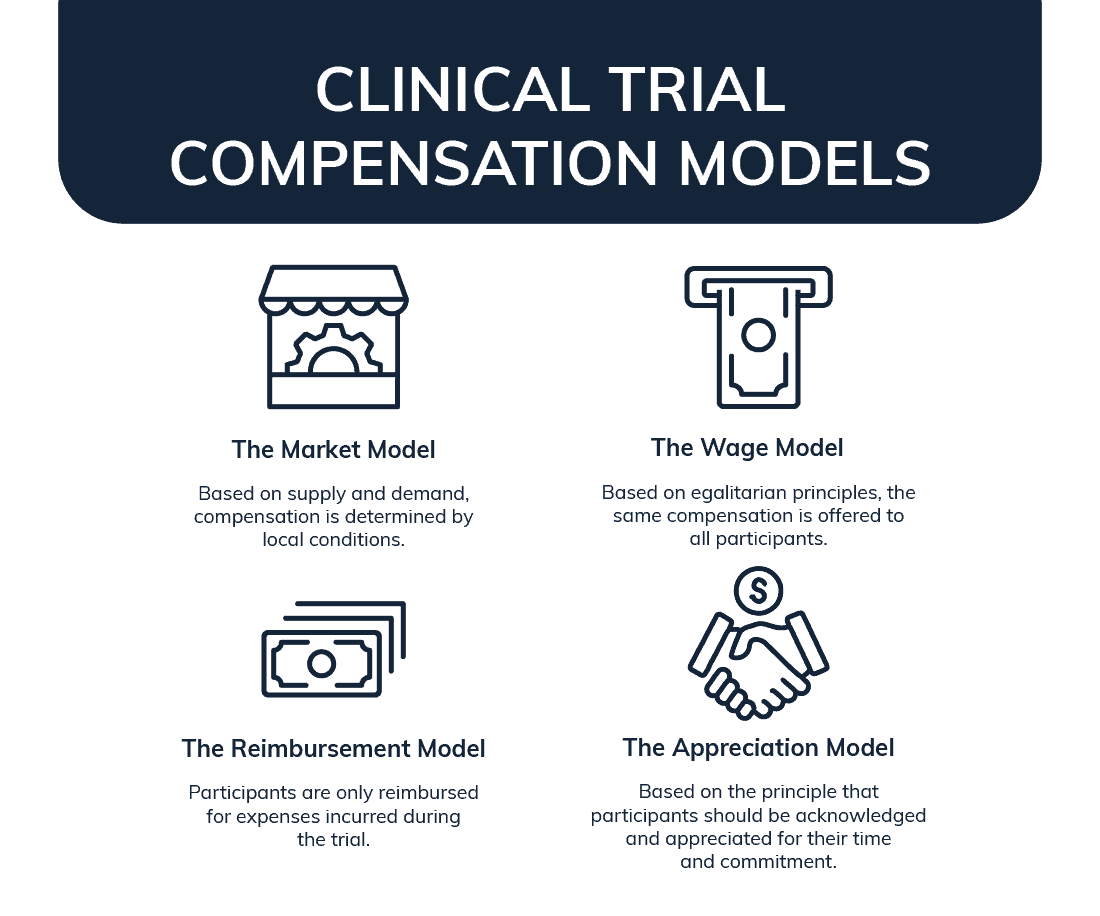
- The market model – based on supply and demand, compensation is determined by local conditions. This includes what, when, and how much is offered along with the ease of finding qualified participants. The more challenging it is to find participants, the larger the sum offered. It’s common for there to be variation between locations in multi-site studies. In some instances, there has been a recorded difference of over $800 offered to participants depending on location.
- The wage model – based on egalitarian principles, the same compensation is offered to all participants. Research participation is treated as a job that requires unskilled labor and compensation rates are typically in line with an hourly minimum wage based on local conditions. In the US, minimum wage varies by state and falls between $7.25 and $11 an hour.
- The reimbursement model – with this model participants are only reimbursed for expenses incurred during the trial. This can include parking, food, and travel costs. In some cases, it may also cover lost wages if a participant takes time off work to be part of the study. With this model participants are asked to track all their pre-approved trial related expenses, and submit receipts in order to be reimbursed.
- The appreciation model – based on the principle that participants should be acknowledged and appreciated for their time and commitment. It’s typically offered at the end of a study and is best used with one of the models previously mentioned. It might include gift cards, thank you notes, small gifts or other meaningful gestures.
While these models can provide guidance, trial sponsors should weigh the pros and cons of each. The models can have a different impact on the length of time it takes to recruit qualified participants, which can have a more substantial effect on study completion. Offering fair compensation can provide incentive for participants to enroll and follow a study through to completion. This can help keep your study on track from both a time and budget perspective.
Conclusion
While there are no hard and fast rules about compensation, it’s an essential consideration for any study. The ethical and practical issues that come with it should be evaluated by trial sponsors early on, and it’s crucial to remember that IRB approval is necessary. Compensation can act as an incentive for participants to enroll, and trial sponsors must ensure it doesn’t cause undue influence that results in poor decision making.
By designing an effective compensation plan based on the industry guidance we’ve outlined, sponsors can boost enrollment, save time, reduce costs and improve study completion rates. The ethical and practical concerns we’ve highlighted can be addressed during the planning stages of a study to help make the process more consistent and transparent.
The use of data can also help when creating a compensation plan. It can give you insight into the number of participants your study will need at each stage of enrollment, allowing you to more accurately forecast expenses. This is especially helpful if you’re recruiting for a rare disease where participants are hard to find and you’re considering offering more money. By understanding these costs upfront there’s less likelihood of unanticipated delays and budget overruns.
Trialfacts has successfully recruited for hundreds of studies using data-informed processes that bring predictability and consistency to enrollment. We can help with your questions related to compensation, the potential impact on enrollment and your study timeline. Schedule a call with us today to find out more about how we can help with your enrollment challenges.