Research Center: Sean N. Parker Center for Allergy & Asthma Research
Location: El Camino Hospital, Tower 4C, 2500 Grant Road, Mountain View, CA 94040
Lead Doctor: Sayantani Sindher, MD
IRB Committee: This study has been reviewed and approved by Stanford Institutional Review Board
Background
 Over the past decade, food allergies have emerged as a significant health issue affecting 3-6% of the population of the United States and researchers have particularly seen an increased prevalence in childhood.
Over the past decade, food allergies have emerged as a significant health issue affecting 3-6% of the population of the United States and researchers have particularly seen an increased prevalence in childhood.
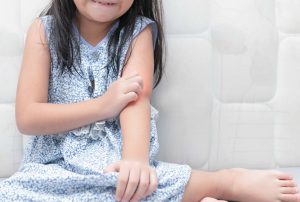
This research study explores the effect of two different moisturizers on adults and children skin that is affected by eczema. Researchers aim to better understand if one kind of moisturizer is better than the other. The two different skin creams to be used include Aveeno Daily Moisturizing Sheer Hydration Lotion (which is available over the counter) and EpiCeram (available with prescription only). Both products are FDA approved and are being used according to labeling.
Adults and children ages from birth to 40 years old who have been diagnosed with eczema are invited to participate. If under 18, each participant must have parental consent to participate. Both the guardian and child are required to attend 4 study site visits over 2 weeks.
Why Participate?
- You or your child may benefit from an improvement in their eczema symptoms
- You or your child will be helping to potentially aid in the advancement and understanding of eczema and help in the development of new approaches for its treatment or prevention.
- You will be helping to advance medical research.
- You will be compensated for participating in the full duration of the study
Your Rights
- If you or your child decide to participate in the study and later feel that you no longer wish to be part of it, you may withdraw at any time.
- Medical records relating to this study and any other information received will be kept strictly confidential, except as required by the law.
- Qualified health professionals will monitor your or your child’s health as it relates to the study.
Who Can Participate?
- Ages 0-40 years old
- Those under 18 whose parents can provide parental consent for participation
- Has been diagnosed with mild, moderate or severe eczema
- Does not have any additional skin conditions
- Able to attend 4 visits at the study site in Mountain View, CA within a period of approximately 2 weeks.